Briefly Explain the Difference Between Polar and Nonpolar Molecules.
Non-Polar Molecule- A molecule in which the individual dipoles cancel each other result in zero net dipole moment is called a. Polar Molecule- A molecule in which the individual dipoles do not cancel each other and results in a net dipole moment is called a polar molecule.
Difference Between Polar And Nonpolar Dielectrics Definition Polarity Examples And Differences
A nonpolar molecule has to have at least one nonpolar bond.

. Polar molecules are asymmetric either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. A polar molecule is a molecule that has oppositely charged regions as a result of polar bonds within the molecule. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out.
So this is or something about simple diffusion. H2 Cl2. Nonpolar molecules are formed when there is quite a little difference in the electronegativities of the atoms forming bonds in the molecule.
Does not have profusion of charges at opposite ends. It is the transport of molecules across the plasma membrane from lower concentration to higher concentration by means of trans membrane protein. H bonds occur in polar bonds.
Does not have electrical poles. Difference between Polar and Nonpolar. Briefly explain why soap is an effective substance for cleaning and disinfecting surfaces citing particular.
Van der waal interactions between nonpolar bonds. Electronegativity is the key factor that differentiates between polar and nonpolar bonds. Electrons are not equally shared.
Therefore not all the molecules with polar bonds are polar. Has dipoles and - regions - region. They are asymmetrical which means the polar bonds do not cancel out as the molecule is lopsided.
Explain why nonpolar molecules usually have much lower surface tension than polar ones. Briefly explain your answer. Explain why nonpolar molecules usually have much lower surface tension than polar ones.
Non-polar molecules are molecules that have no oppositely charged regions and have no net dipole moment. The difference between polar molecules and nonpolar molecules. Which statement is true about polarity.
Ion Induced Dipole Interactions In this type of interaction a non-polar molecule is polarized by an ion placed near it. This animated lecture is about polar molecules and nonpolar molecules in chemistry. Size of the polar molecule The size and charge of an ion 3.
In polar molecule all the bonds collectively should produce a polarity. Non-polar molecules are distributed in a symmetrical manner and do not contain abundant electrical charges that are attached on them. All molecules with polar bonds are polar.
A polar molecule is formed between two dissimilar atoms but a non polar molecule is formed between similar atoms. Briefly explain the difference between polar and nonpolar molecules. Though a molecule has polar bonds it does not make the molecule polar.
H bonds occur in polar bonds. The key difference between polar and nonpolar solvents is that polar solvents dissolve polar compounds whereas nonpolar solvents dissolve nonpolar compounds. The greater the electronegativity difference between two atoms in a bond the less polar the bond.
The shape of a molecule is important in determining the molecular polarity. Area with lower electronegativity value. In chemistry it is the charge separation in a molecule that has atoms or groups of atoms with different electronegativity.
Polar molecules occur when there is an electronegativity difference between the bonded atoms. So in the question we have to briefly explain. If the molecule is symmetric and all the bonds are similar then the molecule may become non polar.
Now after this the next point of differentiation is that transports small and non polar particles. A polar molecule is formed between two dissimilar atoms but a non polar molecule is formed between similar atoms. One end of molecule has positive whereas the other end has negative charge.
How is the polarity of a molecule related to its properties. In polar molecules the difference in electronegativity among the bonding atoms results in the polarity of bonds on the other side. How does polarity affect physical properties.
Explain why soap is more effective than hand sanitizer in protecting individuals from bacterial and viruses. In non-polar molecules the difference of. Draw the chemical structure of soap and label the polar and nonpolar parts.
How Do Polar and Nonpolar Molecules Interact With Each Other. The polarity of a compound refers to the property of having poles. The main difference between polar molecules and nonpolar molecules lies in the arrangement of atoms in the molecule.
O It has a slight positive charge on one side and slight negative charge on the other side. Explain the difference between nonpolar molecules and polar molecules. Non polar molecules are symmetric with no unshared electrons.
Find step-by-step Chemistry solutions and your answer to the following textbook question. Covalent molecules that have atoms with the same electronegativity values. Area with a higher electronegativity value closer to F region.
Marisa Alviar-Agnew Sacramento City College Henry Agnew UC Davis Back to top. Explain why soap is more effective than hand sanitizer in protecting individuals from. Also I will teach you about how to identify a polar and nonpolar molecul.
Contributors and Attributions StackExchange thomij. Covalent molecules that have regions with different electronegativity values. The non-polar molecules upon obtaining a charge behave as induced dipoles.
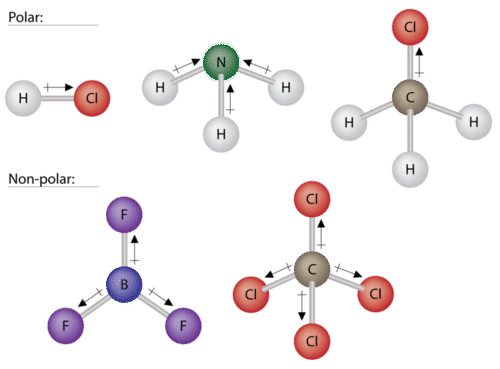
5 3 Polarity And Intermolecular Forces Chemistry Libretexts

Polar And Nonpolar Molecules Youtube

Image Result For Polar Vs Nonpolar Molecules Covalent Bonding Chemical Bond Study Chemistry
Comments
Post a Comment